Lipoprotein A in Cardiovascular Disease

Lipoprotein a is a specific type of lipoprotein particle. Both epidemiological and clinical studies have shown that lipoprotein a plays an important role in the development of cardiovascular disease and is an important risk factor for cardiovascular disease.
Structure of Lipoprotein A
Lipoprotein a is a low-density lipoprotein-like particle whose apolipoprotein B100 is covalently bound to cell surface apolipoprotein A via disulfide bonds. Lipoprotein a levels are almost entirely determined by genes, and the lipoprotein a gene encodes Apo A.

ApoA is a highly polymorphic glycoprotein with larger particles than ApoB and is almost exclusively synthesized and secreted by the liver. The composition of ApoA contains several fibrinogen-like kringleiv structural domains (KIV), a fibrinogen-like kringle V structure, and an inactive protease region. ApoA differs from fibrinogen in kringle type and copy number. Due to expansion and differentiation, 10 different types of KIVs are contained . All these KIVs are composed of specific amino kringle. Among the KIV isoforms of apolipoprotein A, KIV2 exhibits high variability in repeat frequency based on the lipoprotein a allele with copy numbers ranging from 2 to 40, which determines the mass and size of the apolipoprotein A molecule. The number of KIV2 repeats and 2 single nucleotide polymorphisms (rs10455872 and rs3798220) together explained approximately 45% of the variation in lipoprotein a levels. The lower the number of KIV2 repeats, the lower the apolipoprotein A and the higher the lipoprotein a level. Differences in the type and copy number of KIV subunits result in heterogeneity in the size of Apo A proteins, which in turn assemble into lipoprotein a of varying sizes. The result is a wide range of clinically detectable lipoprotein a levels, with inter-individual variations of up to 1000-fold.
Synthesis and Metabolism of Lipoprotein A
Lipoprotein a is encoded by the lipoprotein genes located on chromosomes 6q26 ~q27, which are the major loci regulating lipoprotein a levels and one of the important loci for the risk of coronary atherosclerotic heart disease. Studies have demonstrated that the liver is the major site of lipoprotein a synthesis and that the half-life and rate of synthesis are inconsistent in different populations. The catabolism of lipoprotein a also takes place mainly in the liver, but the pathways are not fully understood. It has been found that there may be several receptors mediating lipoprotein a metabolism and clearance, such as LDL-C receptor-related protein, very low density lipoprotein cholesterol receptor, and group B type I scavenger receptor, etc. The ability of group B type I scavenger receptor to clear lipoprotein a is very important. The more lipoprotein a is taken up by macrophages, the more it is converted into foam cells, which eventually accelerates the formation of atherosclerosis. In addition to the above receptors, desialic acid glycoprotein receptors and Megalin receptors are also involved in lipoprotein a metabolism. In addition, patients with kidney disease have higher lipoprotein a levels than healthy people, suggesting that the kidney may play an important role in lipoprotein a clearance process.
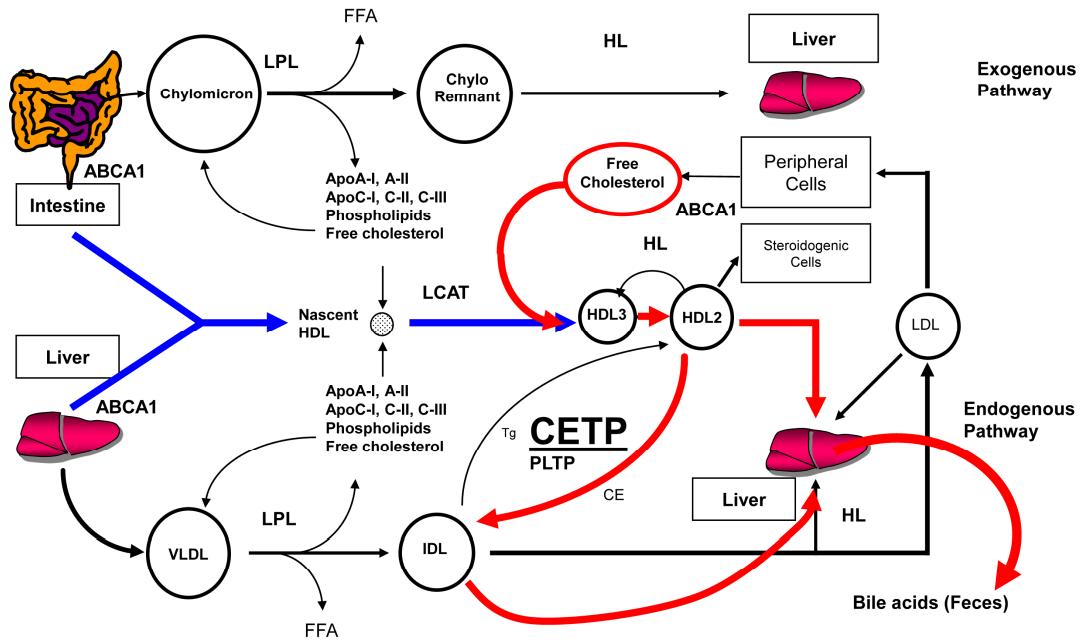 Schematic representation of lipoprotein metabolism (Choi et al., 2017)
Schematic representation of lipoprotein metabolism (Choi et al., 2017)
Mechanism of Action of Lipoprotein A
The primary physiological mechanisms of lipoprotein a function are unknown, but affect cardiovascular health in multiple ways.
The lipoprotein a complex contains 1 cholesterol-adenylate-low-density lipoprotein-like particle that can block arteries and lead to luminal narrowing. The primary carrier of the pro-inflammatory molecule in ApoA is known as oxidized phospholipid, which is a biomarker of pro-atherosclerotic and pro-inflammatory responses. In addition, oxidized phospholipids covalently bind to KIV10 of ApoA through the lysine binding site and can exacerbate atherosclerosis. Increased plasma levels of oxidized phospholipid-A and oxidized phospholipid-Apolipoprotein B are associated with the risk of aortic stenosis.
![Mechanisms linking lipoprotein(a) (Lp[a]) to thrombosis and atherosclerosis](https://lipidomics.creative-proteomics.com/upload/image/Lipoprotein-A-in-Cardiovascular-Disease-3.jpg) Mechanisms linking lipoprotein(a) (Lp[a]) to thrombosis and atherosclerosis (Spence et al., 2012).
Mechanisms linking lipoprotein(a) (Lp[a]) to thrombosis and atherosclerosis (Spence et al., 2012).
Lipoprotein A causes massive proliferation of vascular smooth muscle cells, increased foam cell formation, necrotic center formation, and proatherosclerotic effects. The structural similarity between apolipoprotein A and fibrinogen makes it possible that apolipoprotein A competitively inhibits fibrinogen activation. This could also explain the potential role of lipoprotein A in increasing the risk of atherosclerotic thrombosis. Since lipoprotein a is also a carrier of cholesterol into the arterial wall, it may be associated with atherosclerosis and thrombosis. Lipoprotein a also enhances the oxidative stress response and is associated with endothelial dysfunction. Since lipoprotein a is rich in platelet-activating factors, its particles may promote platelet aggregation. In conclusion, lipoprotein a particles contain apolipoprotein A in addition to the atherogenic component of LDL-C, and thus may be more atherogenic than LDL-C particles in terms of atherogenic thrombosis.
The Role of Lipoprotein A in Cardiovascular Disease
The pathogenicity of lipoprotein a can be broadly classified as pro-atherosclerotic, pro-inflammatory, and pro-thrombotic. Body lipoprotein a levels can reach 0 ~> 10 000 mg/L. Although it is generally accepted that elevated lipoprotein a levels are a risk factor for atherosclerotic cardiovascular disease, recommendations for risk level thresholds vary. The European Atherosclerosis Society recommends that lipoprotein a levels should be less than 500 mg/L for preventive purposes, and the Canadian Cardiovascular Society recommends 300 mg/L as an appropriate threshold. Elevated plasma lipoprotein a levels increase the risk of acute myocardial infarction, ischemic stroke, aortic valve disease, and peripheral arterial disease.
Creative Proteomics offers lipoprotein content analysis services to facilitate cardiovascular disease research and ensure healthy development. Based on LC-MS/MS platform, we can help our customers with lipidomics analysis and facilitate basic research.
References
- Choi, H. Y., Hafiane, A., Schwertani, A., & Genest, J. (2017). High-density lipoproteins: biology, epidemiology, and clinical management. Canadian Journal of Cardiology, 33(3), 325-333.
- Spence, J. D., & Koschinsky, M. (2012). Mechanisms of lipoprotein (a) pathogenicity: prothrombotic, proatherosclerotic, or both?. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(7), 1550-1551.
- Questions and Answers
- Opinion
- Story/Motivational/Inspiring
- Technology
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film/Movie
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News
- Culture
- War machines and policy

