Key Market Dynamics Shaping the Future of In Vitro Diagnostics in Europe
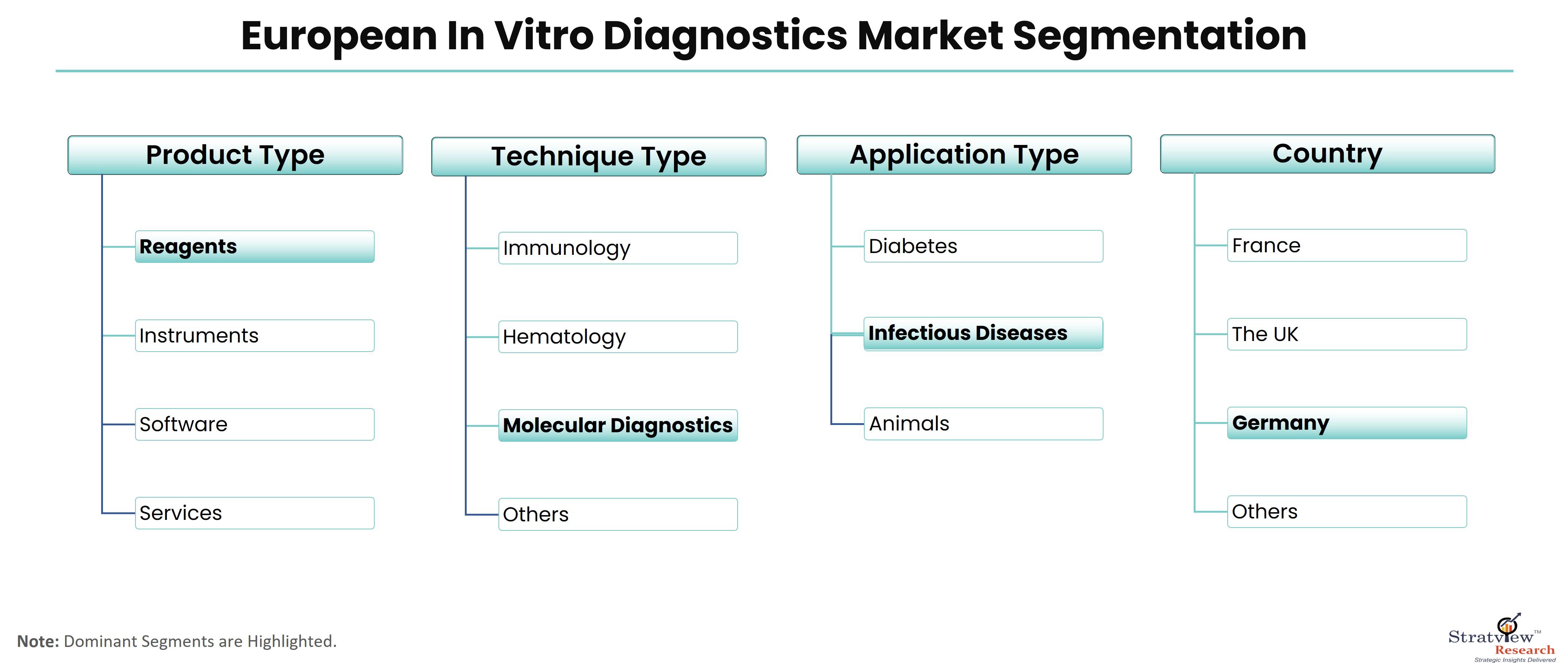
The In Vitro Diagnostics market in Europe is undergoing rapid transformation, driven by a multitude of dynamic factors. These dynamics are reshaping the industry landscape, offering new opportunities and posing fresh challenges for stakeholders. This article explores the key market dynamics shaping the future of IVD in Europe.
According to Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
Technological Advancements
Technological innovation is the cornerstone of growth in the European IVD market. Breakthroughs in molecular diagnostics, such as next-generation sequencing (NGS) and polymerase chain reaction (PCR), are revolutionizing the ability to diagnose and monitor diseases with unprecedented accuracy. Additionally, advancements in digital pathology and point-of-care testing (POCT) are making diagnostics faster and more accessible, thereby enhancing patient care.
Aging Population and Rising Chronic Diseases
Europe’s aging population significantly impacts the demand for IVD products. The prevalence of chronic diseases such as diabetes, cardiovascular conditions, and cancer is increasing as the population ages. Early detection and continuous monitoring of these diseases are crucial for effective management, leading to higher utilization of diagnostic tests. This demographic shift is a major driver for the growth of the IVD market.
Regulatory Environment
The regulatory landscape in Europe is evolving with the implementation of the In Vitro Diagnostic Regulation (IVDR). This regulation aims to ensure the safety and efficacy of IVD products by imposing stringent requirements for testing and certification. While navigating these regulations can be challenging, compliance fosters innovation and ensures high-quality diagnostic solutions. Companies that successfully adapt to these changes can gain a competitive advantage by offering reliable and compliant products.
Integration of Artificial Intelligence and Big Data
Artificial intelligence (AI) and big data are transforming the IVD sector by enhancing diagnostic accuracy and operational efficiency. AI algorithms can process vast amounts of data to identify patterns and predict disease outcomes more accurately than traditional methods. In fields like oncology and genomics, AI-driven tools are crucial for precision diagnostics and personalized treatment plans. The integration of AI and big data is expected to continue driving innovation and efficiency in the IVD market.
Increasing Adoption of Point-of-Care Testing
Point-of-care testing (POCT) is gaining traction due to its ability to deliver rapid results outside traditional laboratory settings. POCT devices are becoming more prevalent in hospitals, clinics, and home care, providing immediate diagnostic information that supports timely clinical decisions. The growing demand for POCT is driven by the need for quick diagnostics in emergency and primary care settings, contributing to the expansion of the IVD market.
Healthcare Infrastructure and Investment
Investments in healthcare infrastructure across Europe are enhancing access to advanced diagnostic technologies. Government initiatives and funding are supporting the modernization of healthcare facilities and the adoption of innovative IVD solutions. These investments aim to improve patient care, reduce healthcare costs, and address the increasing burden of chronic diseases.
Personalized Medicine
The shift towards personalized medicine is significantly impacting the IVD market. Personalized medicine tailors treatments based on an individual’s genetic profile and specific disease characteristics, requiring precise and reliable diagnostic tools. IVD products are integral to this approach, providing the necessary data to guide targeted therapies. The trend towards personalized medicine is expected to drive the development of specialized diagnostics and companion tests.
Conclusion
The future of the In Vitro Diagnostics market in Europe is being shaped by technological advancements, demographic changes, regulatory shifts, and the increasing integration of AI and big data. The rising adoption of point-of-care testing and significant investments in healthcare infrastructure are further propelling the market. As the demand for precise and efficient diagnostics continues to grow, the European IVD market is poised for robust expansion. Stakeholders must continue to innovate and adapt to these dynamic market forces to capitalize on the opportunities and navigate the challenges ahead.
- Questions and Answers
- Opinion
- Story/Motivational/Inspiring
- Technology
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film/Movie
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News
- Culture
- War machines and policy

